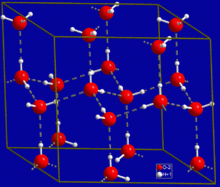
Hydrogen bonds in hexagonal ice.
Every fall semester, nearly a thousand bright-eyed freshmen take their seats for Chemistry 111, and told that throughout the course they will learn the story of the electron. It makes for a great cohesive semester (though famously challenging for the students), as the professors move through electron properties, atomic structure, and molecular orbitals to arrive at chemical bonds, described by bonding researcher Alexander Boldyrev as ‘the heart of chemistry.’ However, while in this format chemical bonding is presented as a finished chapter of established science, its nuances and variations remain as murky to the world’s experts as they do to Chem 111 students. So much so that there is now an established International Conference on Chemical Bonding, created in an attempt to define bonding as a whole, as well as work on the age old question: “What is a hydrogen bond?”
Hydrogen bonds are one of the most important concepts in chemistry, to the point that during one of my undergraduate classes it became a running joke to answer any unknown test questions with ‘hydrogen bonding.’ They are responsible for the crystalline structure of ice, holding our DNA together, protein structure, natural enzymatic reactions that occur as part of our regular biochemistry, as well as enzymatic reactions that occur from pharmaceuticals. Yet the essence of the bond continues to elude researchers. Many of us have learned that a hydrogen bond is the electrostatic interaction between an electronegative atom (fluorine, oxygen, nitrogen) and a hydrogen atom that is bound to an electronegative atom, a simple example being the bonds that form in water. However, whether it can be considered a bond or merely an electrostatic interaction is still the subject of intense debate.
Similarly, the bonds between electrons are also being called into question, as we are still trying to figure out what actually holds our world together. Typically, electronic bonds are described as electrons shared between atoms. Sometimes they are shared equally (covalent bonding), and in other instances less equally between the two atoms (ionic bonding). However in his research, Boldyrev questions this canonical view, stating, “For me, a chemical bond is no longer an electron pair sitting between two atoms. It is more simply a two-electron blob that can connect two, three, five, or more atoms. We allow the pairs to run over whatever part of the molecule they want to occupy.”
Given the current work being done on chemical bonding despite its reputation for being ‘old’ and ‘over,’ the American Chemical Society publication C&EN asked chemists focused on chemical bonding to answer: “What do you think a chemical bond is in reality?” in essence asking what is the molecular glue that holds us together? Surprisingly, the answers were much more abstract than one would expect from a scientist devoting his/her work to understanding the nature of these bonds, however most came to the same consensus that a chemical bond is an attraction between two atoms. Specialist Akire Sekiguchi describes the interaction as “a handshake between two atoms: each atom stretches its ‘arm’ (electron) toward the other, and when these two ‘arms’ (electrons) meet, then the ‘handshake’ (chemical bond) takes place.”
Though the details of this variety of research are not intuitively comprehensive, this is perhaps one of the most applicable and underappreciated areas of chemistry, as understanding these bonds both in organic and inorganic compounds is essential to understanding the world we live in. Without these bonds we would not be cohesive beings, we would not be capable of forming the microscopic protein building blocks that make up our bodies. Because of this, Boldyrev has stated, “I don’t think chemists are abandoning the concept of chemical bonding anytime soon.” I, for one, am looking forward to the results of the 2015 International Conference on Chemical Bonding.
